Long-term slow release, one shot last for seven days
No immunosuppression, can be used during viral vaccination
Good tolerance at the injection site
Features
1. Long-term slow release, one shot last for seven days
The ceftiofur hydrochloride crystal combined with the new formulation process ensures the slow release of the active ingredient, and the blood concentration of the drug exceeds 90%. The minimum inhibitory concentration of respiratory pathogens is 168 hours (7 days).
2. Broad spectrum and efficient sterilization, less resistance
It has ideal effects on infections such as Pasteurella multocida, Pasteurella hemolyticus, Actinobacillus pleuropneumoniae, Haemophilus parasuis, Streptococcus, Staphylococcus, Escherichia coli and Salmonella.
Table 1 The minimum inhibitory concentration of Saifeixin in Shandong province
Bacteria (several isolates from Shandong pig farms) | MIC50(ug/ml) | MIC90(ug/ml) | MIC range(ug/ml) |
Streptococcus | ≤0.02 | ≤0.03 | ≤0.02~0.03 |
Haemophilus parasuis | ≤0.03 | ≤0.12 | ≤0.03~0.12 |
Actinobacillus pleuropneumoniae | ≤0.06 | ≤0.15 | ≤0.06~0.15 |
Pasteurella multocida | ≤0.03 | ≤0.06 | ≤0.03~0.06 |
Pasteurella hemolyticus | ≤0.01 | ≤0.03 | ≤0.01~0.03 |
Escherichia coli | ≤0.27 | ≤0.46 | ≤0.27~0.46 |
Staphylococcus aureus | ≤0.59 | ≤0.78 | ≤0.59~0.78 |
Note:MIC= minimum inhibitory concentration;MIC50=Minimum inhibitory concentration of 50% bacteria inhibited;MIC90=Minimum inhibitory concentration of 90% bacteria inhibited 90%
3. Safety
a) The injection site is well tolerated;
b) No adverse reactions have been observed in the injected pigs;
c) No immunosuppression, can be used before and after inoculation of viral vaccine;
d) After the bottle cap is opened, it is still valid when stored at low temperature for 12 weeks.
4. Bioequivalence
Tests have shown that Saficin and its similar products are bioequivalent in pigs. The mean area AUC0-∞ under the plasma concentration-time curve was 369.2±123.7 ug/ml and 385.1±118.9 ug/ml, respectively.。
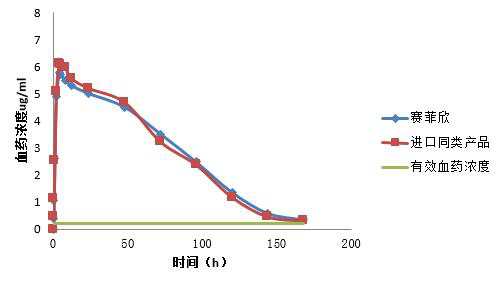
Figure1. Bioequivalence: The area under the curve of the test preparation (AUC) and the peak concentration of blood (Cmax) reached between 80 and 125% of the original drug.
It can be seen from Fig. 1 that the average AUC0-∞ area under the blood concentration-time curve of Saifisin and the original drug is 369.2±123.7 ug/ml and 385.1±118.9 ug/ml, respectively, and the relative bioavailability is 97.5%. It can be considered that Safeixin is bioequivalent to the original drug.
Table 2 Comparison of Saifin and similar preparations
product items | Saifeixin | Common ceftiofur | Imported product |
Excipient | Imported | Domestic (with impurities) | Imported |
Formulation process | American emulsification process | No formulation process | Original research process |
Effective time | 168 h | 36-72h | 168h |
Injection stimulation | No irritation at the injection site | Strong stimulus | No irritation at the injection site |
Storage | Unused low temperature for 12 weeks | short time | Unused low temperature for 12 weeks |
Convenience | Convenient | inconvenient | Convenient |
Saifeixin's application, case on the farm
A pig farm in Shandong was weaned at 23 days of age. Piglets after weaning showed symptoms of porcine respiratory disease such as rough hair, cough, weight loss, and asthma. The incidence rate was 17%. In the test group, the piglets were injected with Saifeixin on the day of weaning, 0.3 ml per head. The results are as follows:
Table 3. Saifeixin test results
Items | Control group | Test group | Difference |
Number of trials | 30 | 51 | +21 |
Average weaning weight(kg) | 7.18 | 7.15 | -0.03 |
Average weight at day 60(kg) | 18.27 | 20.49 | +2.22 |
Total weight gain during nursery(kg) | 11.09 | 13.34 | +2.25 |
Average daily weight gain(g/heda) | 299.73 | 360.54 | +60.81 |
Survival rate of piglets during cNursery(%) | 92 | 100 | -8 |
FCR during nursery | 1.62 | 1.35 | -0.27 |
* Compared with the control, the average daily weight gain increased by 61 grams; the feed-to-weight ratio decreased by 0.27; the survival rate increased by 8%.
Safeixin’s usage
u One dose only: intramuscular injection at the neck, 5mg/kg body weight, or 1ml/20kg body weight.
u No more than 2ml per injection site, more than 40kg pigs need more injection sites.
Safeixin medication procedure
u For prevention, group health
According to the characteristics of the disease of the farm, it is usually 2 to 3 days before the onset of the disease.
1)7~10 days of age: intramuscular injection 0.2m l/head;
2)Nursery piglets: intramuscular injection 0.3ml/head on weaning day; or intramuscular injection 0.5ml/head 2 weeks after weaning;
3)On PRRS unstable farm: 1 to 2 weeks after weaning, injection of Saifeixin 1ml/20kg body weight.
u For individual treatment
When the affected pig is diagnosed as a bacterial infection (or mixed infection) such as streptococcus suis, haemophilus parasuis, actinobacillus pleuropneumoniae or pasteurella multocida, it is immediately injected with 1 ml/20 kg body weight of Safeixin.
Others
Traits: gray to gray yellow translucent suspension
Specification: 50ml: 5g
Withdrawal period: pig 4 days
Package: 50ml/bottle×20 bottles/box